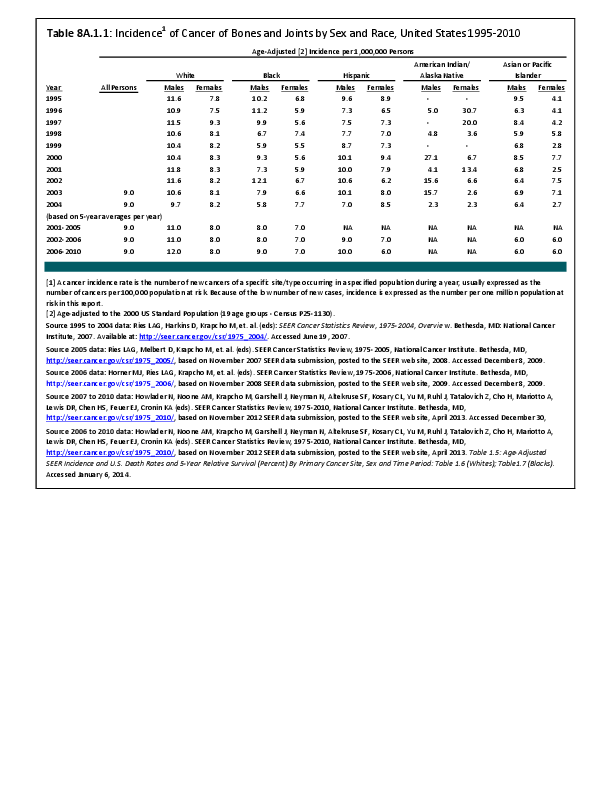
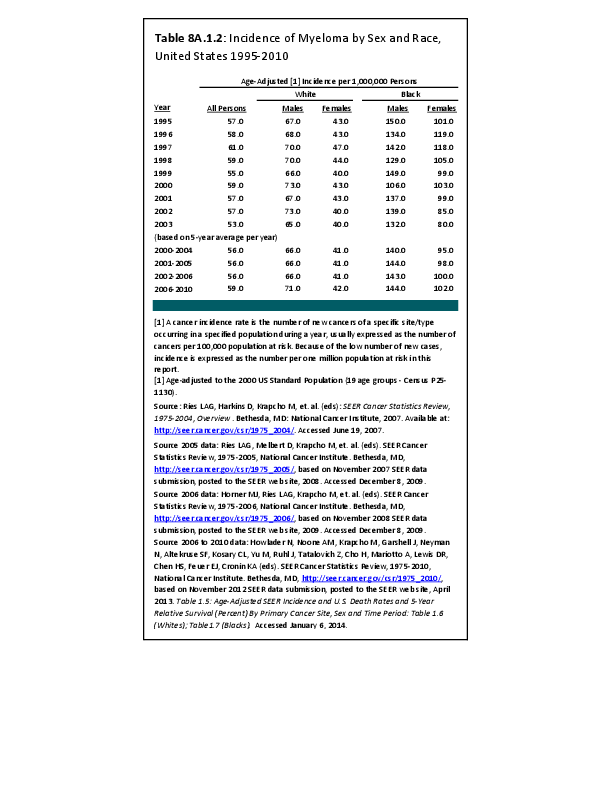
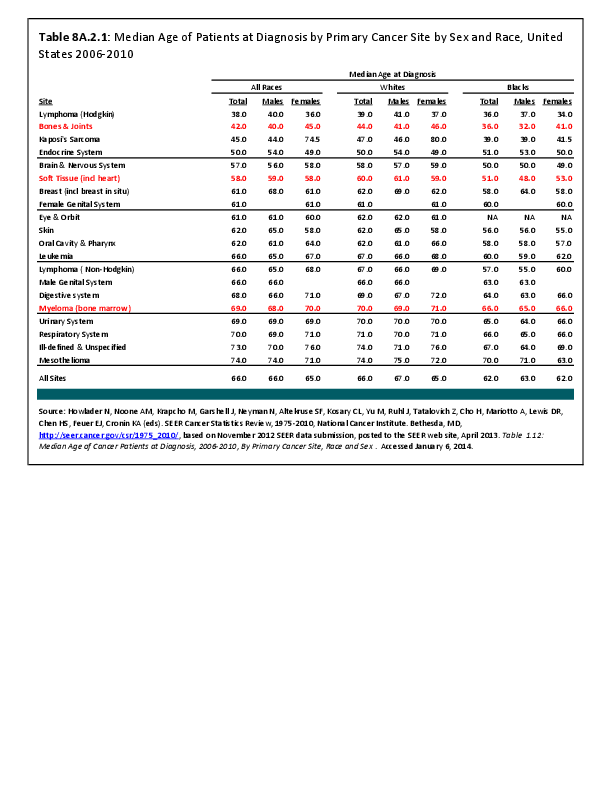
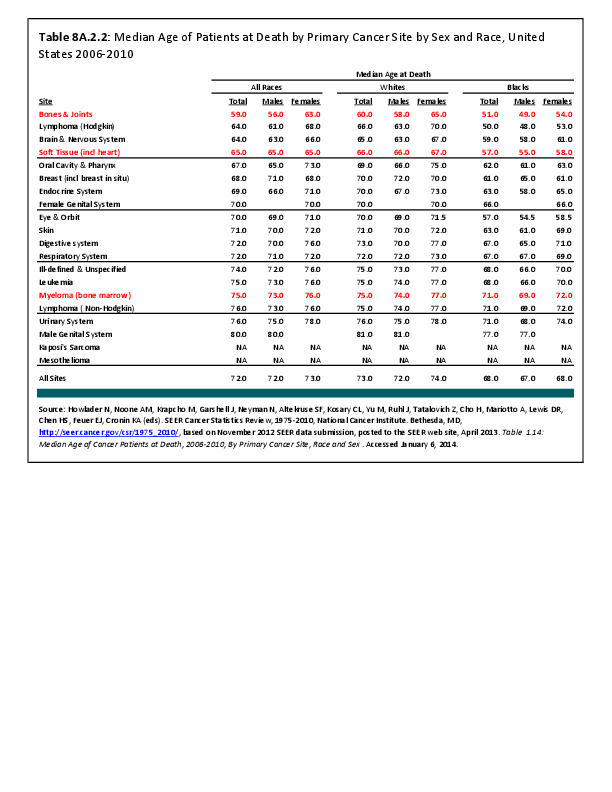
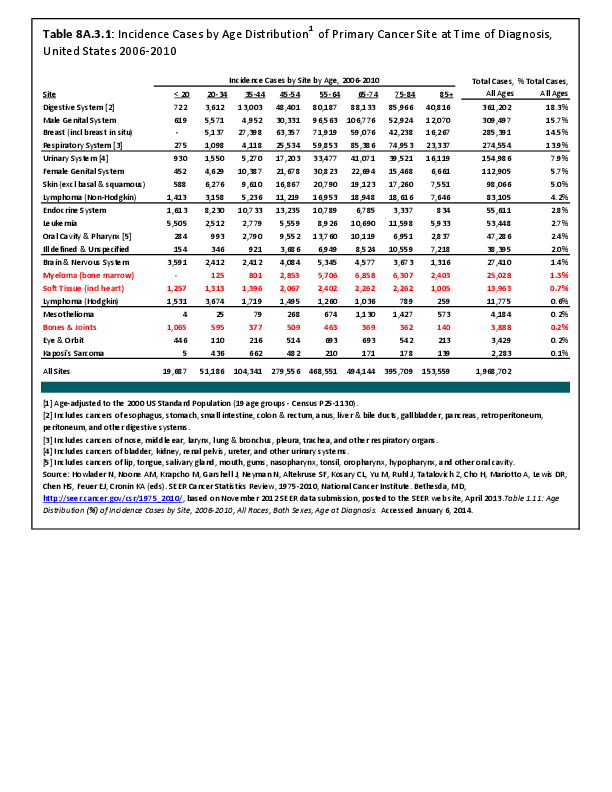
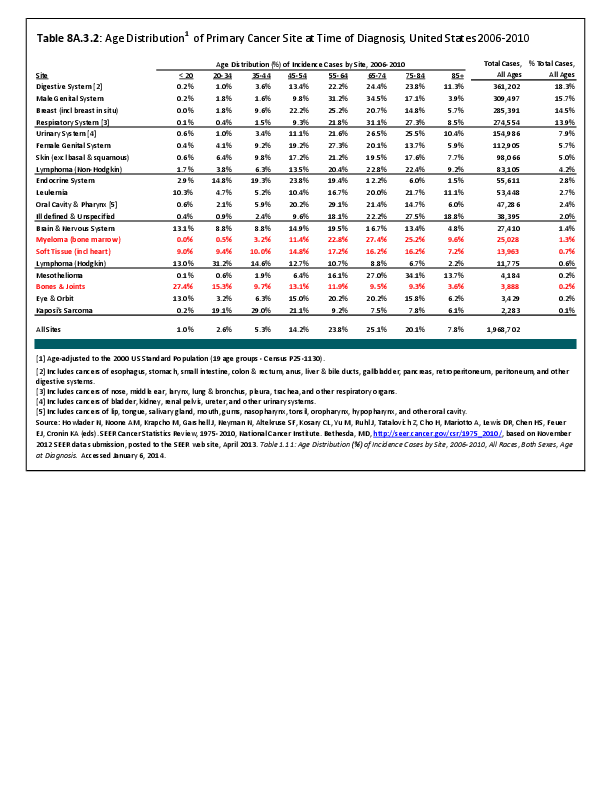
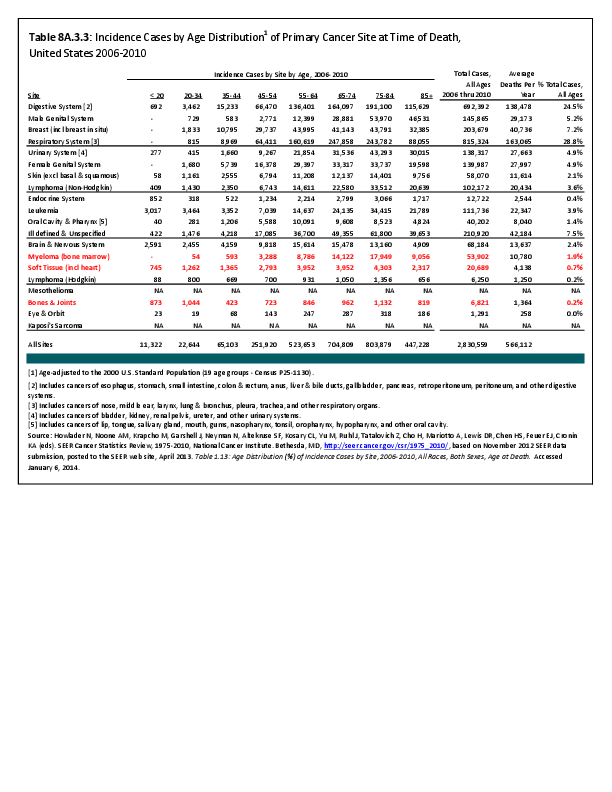
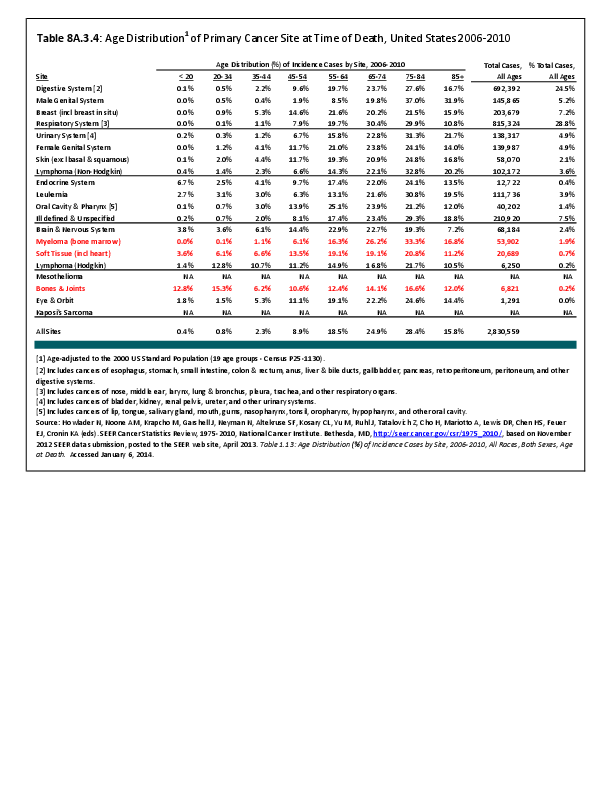
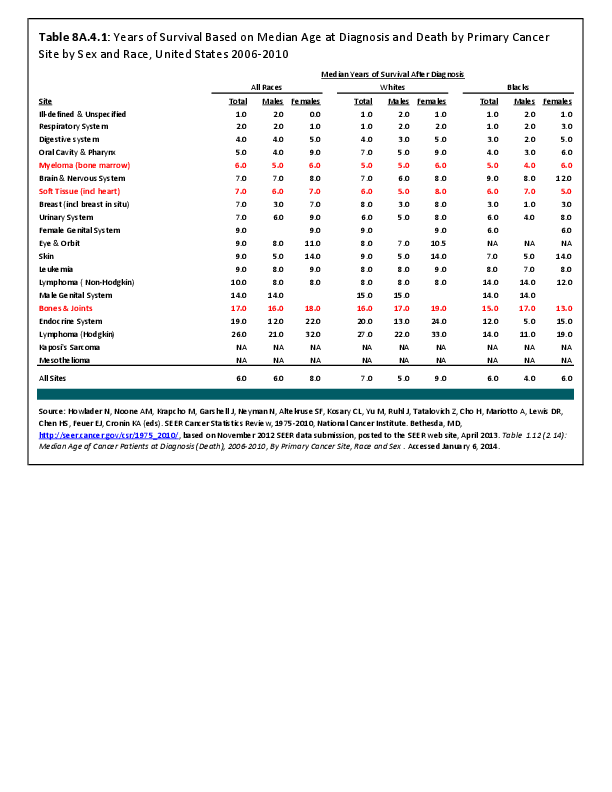
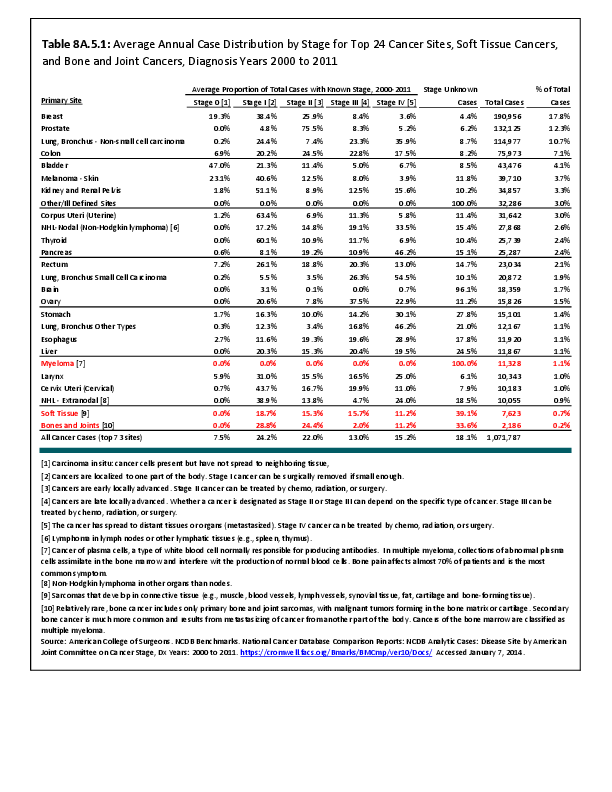
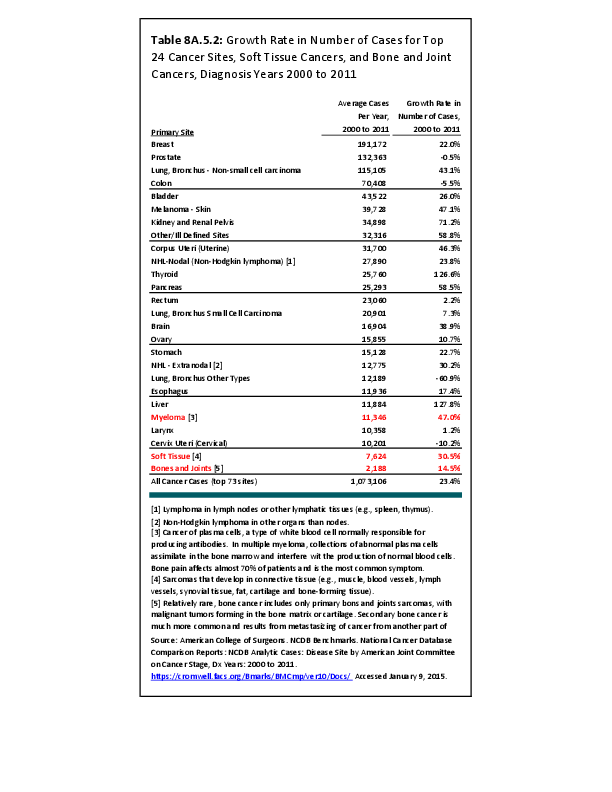
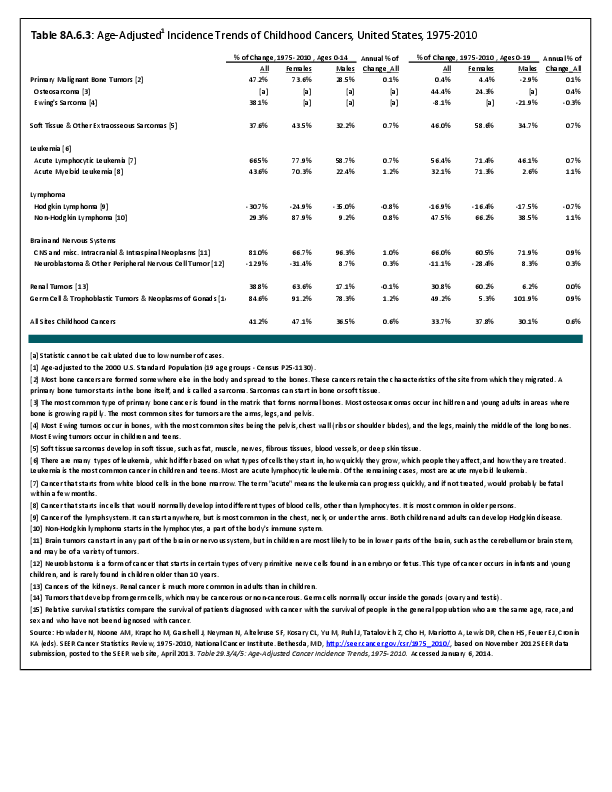
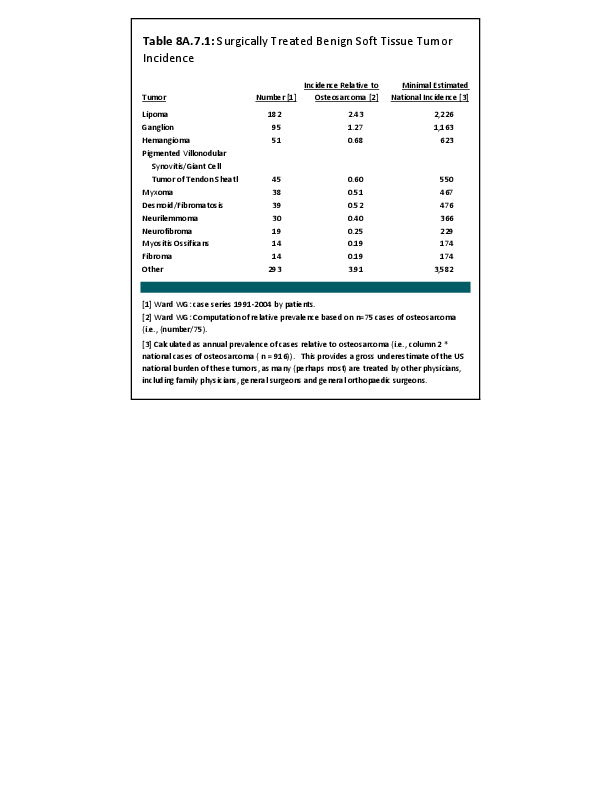
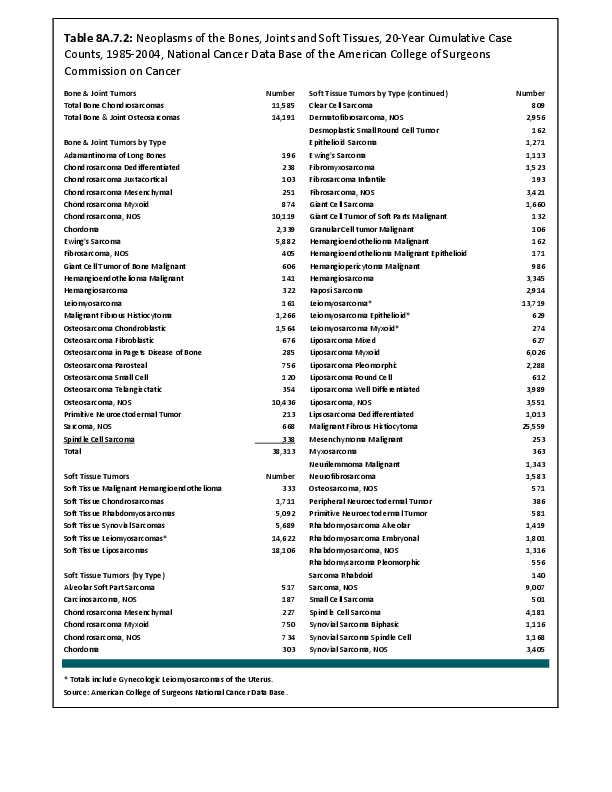
Almost all cancers have preferential sites to which they spread or metastasize, resulting in secondary cancers. Secondary bone cancer is much more common than primary bone cancers, and result in great morbidity and pain. The three most common sites of cancer metastasis are lung, liver, and bone. The skeleton is the most common organ affected by metastatic cancer, and the site of disease that produces the greatest morbidity. The most commonly encountered cancers that readily and frequently spread to bone are cancers of the breast, lung, kidney, prostate, gastrointestinal tract, and thyroid gland. The incidence of bone metastases in lung cancer patients is approximately 30% to 40%, and the median survival time (MST) of patients with such metastases is 6 to 7 months.1 At postmortem examination, 70% of patients dying of breast and prostate cancer have evidence of metastatic bone disease. Cancers of the thyroid, kidney, and bronchus also commonly give rise to bone metastases, with an incidence at postmortem examination of 30% to 40%.2 Brain and ovarian cancers rarely spread to bone. Many other cancers have intermediate rates of spread to bones.
A tumor formed by metastatic cancer cells is called a metastatic tumor or a metastasis. The cancer cells in their new metastatic site closely resemble the original or primary cancer from which the cancer initially arose. For example, breast cancer that spreads to the bone and forms a metastatic tumor is still considered metastatic breast cancer, not true bone cancer. It will still look like breast tissue and breast cancer when it is inspected or viewed under a microscope. Many lay people will now refer to it as bone cancer, but to the physician, bone cancer implies a cancer that started or originated in the bone, such as osteosarcoma, Ewing sarcoma, or myeloma, as discussed above.
Metastatic bone disease complications are termed skeletal-related events (SREs). SREs include pain, pathologic fracture, vertebral deformity and collapse, spinal cord compression, and hypercalcemia (overabundance of calcium in the blood) of malignancy. These complications result in impaired mobility and reduced quality of life (QOL) and have a significant negative impact on survival.2 In addition, metastatic disease may remain confined to the skeleton, with the decline in quality of life and eventual death almost entirely due to skeletal complications and their treatment.
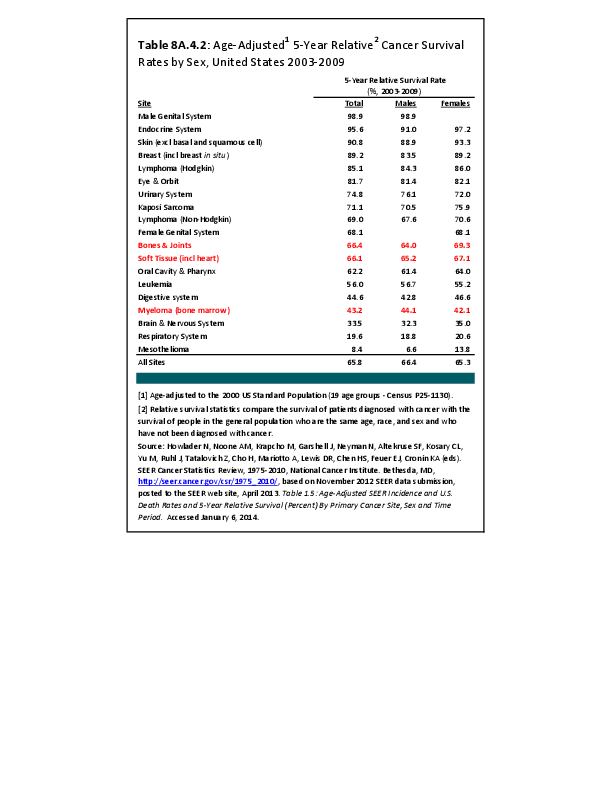
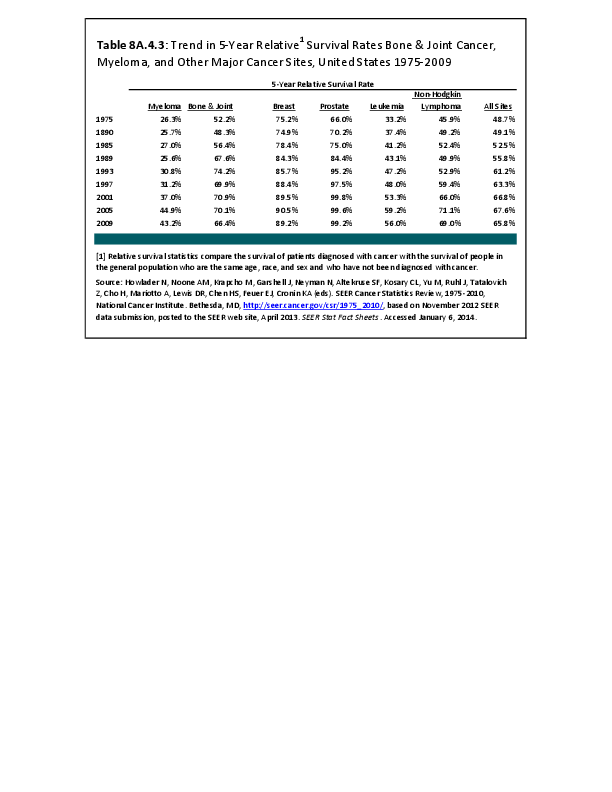
The prognosis of metastatic bone disease is dependent on the primary site, with breast and prostate cancers associated with a survival measured in years compared with lung cancer, where the average survival is only a matter of months. Survival rates for secondary bone cancer depend on patient factors such as age, overall health, treatment, and response to treatment. However, due to the advanced stage of cancer that has spread, survival rates are much lower than for primary cancer without such spread.
The fundamental treatment for bone metastasis from advanced cancer is disease control by systemic chemotherapy and radiation of the bone lesions. Prevention and treatment of bone metastases is highly dependent on an effective treatment being employed against the primary cancer. As a direct treatment for bone metastases themselves, radiation therapy, surgery, and bisphosphonates are the mainstays of treatment. Intravenous bisphosphonates, such as zoledronic acid have been shown to prevent or reduce pathologic fractures and may reduce these costs.3 With FDA approval of the use of bisphosphonate medications to prevent such fractures in 1995, the incidence of fractures in treated patients has significantly decreased. The fracture rates reported in cases of metastatic disease and myeloma have been demonstrated in multiple studies to markedly diminish (roughly a 50% reduction in fracture rates in many studies). Although the tumors still metastasize to the bone, the associated bone destruction and consequent pathologic fracture rate is markedly less. The bisphosphonate medications work by interrupting a biochemical pathway required for bone breakdown by osteoclasts, the cells that normally remove bone in the process of bone remodeling. This bone breakdown step is overactivated in the presence of bony metastases, causing bone loss, bone destruction, and ultimately fractures from the weakening of the bone. Thus, the introduction of bisphosphonate medication has been a major advance over the past 20 years, one that had significant impact on the health of those with myeloma and metastatic cancer to the bone by preventing bone destruction, and thereby preventing bone weakening and subsequent fractures.
- 1. Tsuya A, Kurata T, Tamura K, Fukuoka M: Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007;57:229-232.
- 2. a. b. Coleman RE: Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12: 6243s-6249s.
- 3. Capalbo S, Delia M, Diomede D, et al: Jaw osteonecrosis associated with use of bisphosphonates and chemotherapy: Paradoxical complication of treatment of bone lesions in multiple myeloma patients. Int J Hematol 2006;83:439-442.
Edition:
- 2014

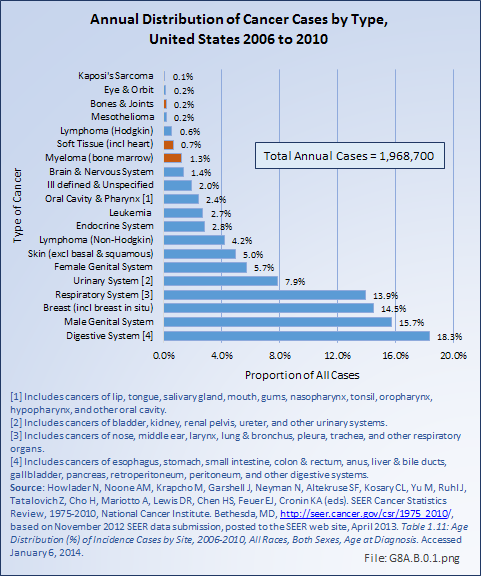
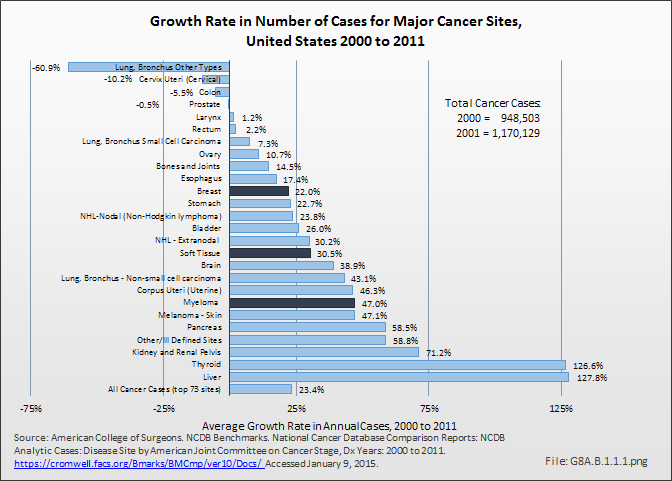
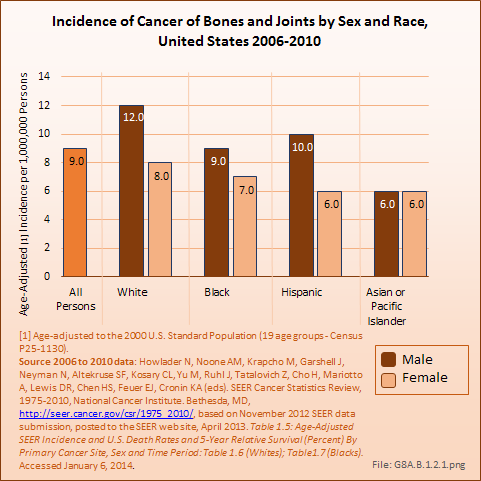
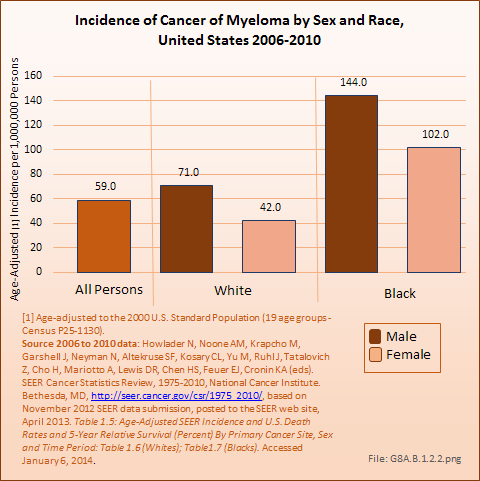
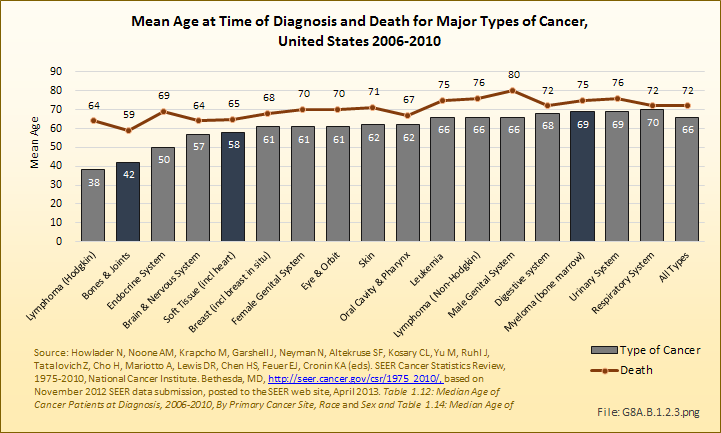
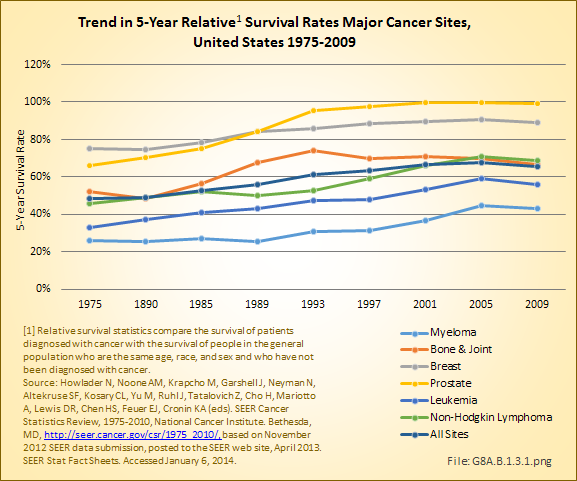
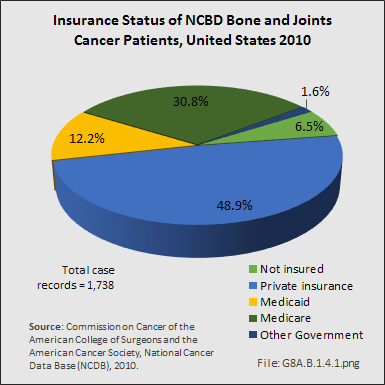
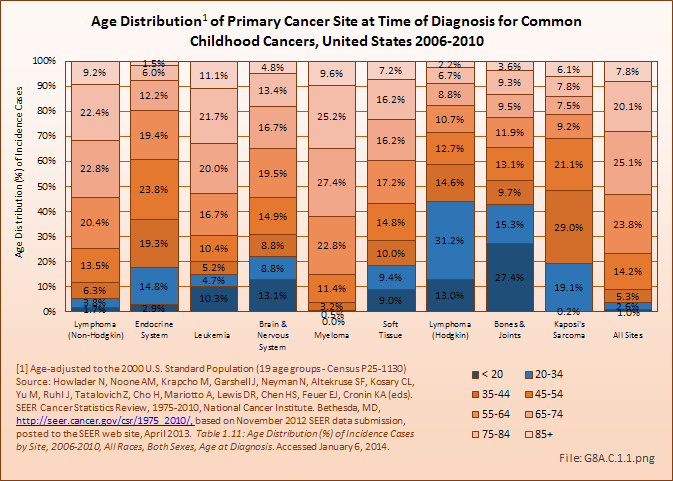
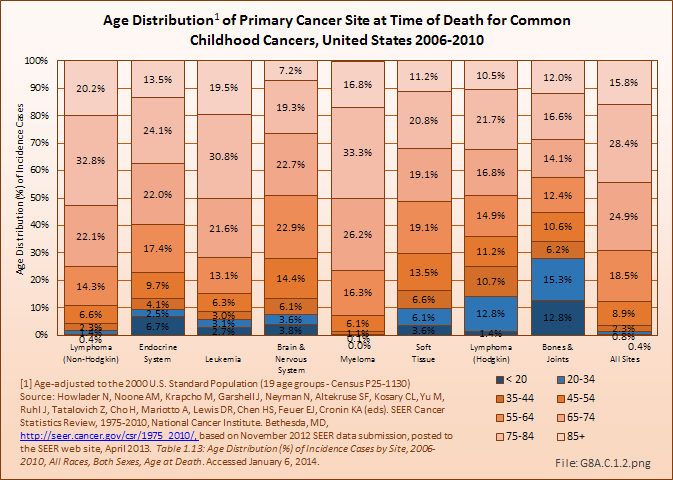
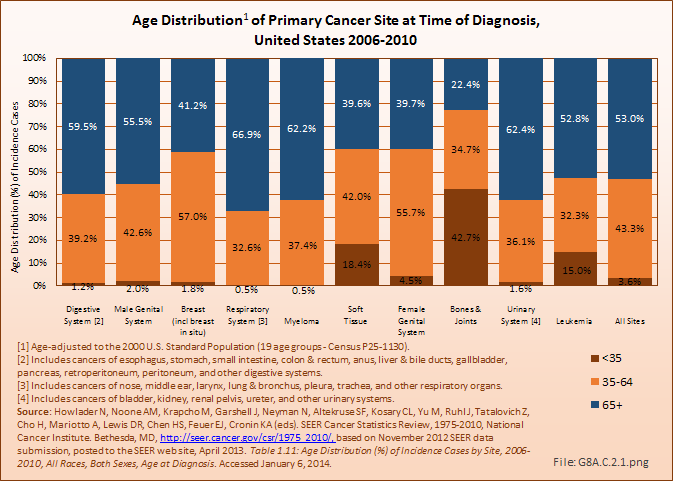
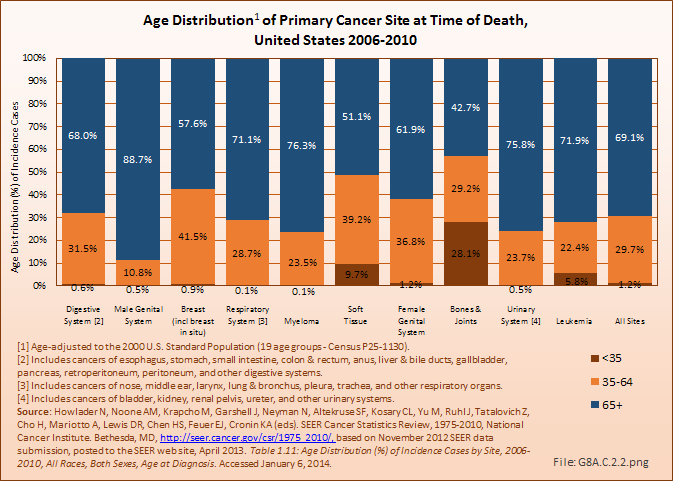
 Download as CSV
Download as CSV