There are three new drugs being tested as potential osteoporosis treatments.
Abaloparatide
Abaloparatide is an analog of parathyroid hormone-related protein (PTHrP) being developed as a potential anabolic agent for osteoporosis treatment. In a phase 2 study, abaloparatide increased BMD at the lumbar spine, femoral neck, and total hip in a dose-dependent fashion. The abaloparatide-induced BMD increases either trended to or were significantly greater than those seen with teriparatide. Phase 3 studies will evaluate fracture outcome and additional safety with this therapy.
Odanacatib
Odanacatib is a selective inhibitor of cathepsin K, a collagenase produced by osteoclasts and responsible for the degradation of bone mineral and matrix. Women receiving odanacatib for five years gained BMD at the spine and hip, and showed bone biomarker changes somewhat different from those seen with bisphosphonates and typical antiresorptive therapies.1,2 Also in contrast with bisphosphonates, after odanacatib discontinuation there was rapid reversal of treatment effects. Although the safety profile has been mild, cathepsin inhibitors have been associated with a scleroderma-like skin lesion. Other potential safety signals are being further investigated.
Anti-sclerostin antibodies
Sclerostin is produced by osteocytes and inhibits anabolic signaling pathway important for bone formation. Rare natural deficiencies in sclerostin result in sclerosing bone diseases, sclerosteosis, and van Buchem disease, which are radiographically characterized by thickened bones, including all long bones. Monoclonal antibodies to sclerostin are being investigated for both osteoporosis treatment and for fracture healing. Preliminary studies suggest a significant increase in BMD with the anti-sclerostin romosuzumab.3 Phase 3 clinical trials evaluating fracture risk protection are underway.
- 1. Eisman JA, Bone HG, Hosking DJ, et al.: Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res 2011 Feb;26(2):242-251. doi: 10.1002/jbmr.212.
- 2. Langdahl B, Binkley N, Bone H, et al.: Odanacatib in the treatment of postmenopausal women with low bone mineral density: Five years of continued therapy in a phase 2 study. J Bone Miner Res 2012 Nov;27(11):2251- 2258. doi: 10.1002/jbmr.1695.
- 3. McClung MR, Grauer A, Boonen S, et al.: Romosozumab in postmenopausal women with low bone mineraldensity. N Engl J Med 2014;370:412-420. doi: 10.1056/NEJMoa1305224.
Edition:
- 2014

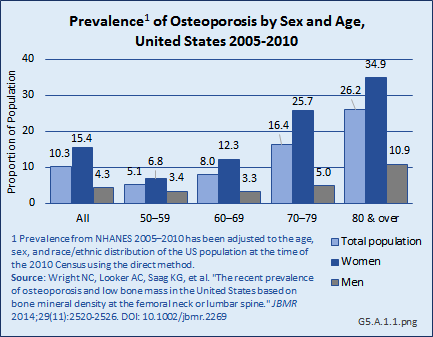
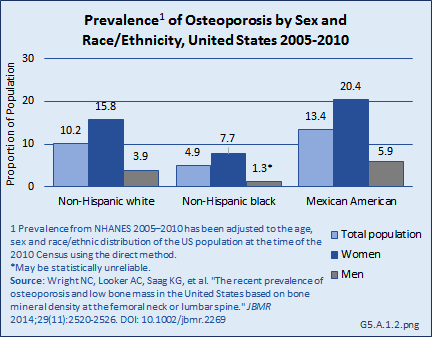
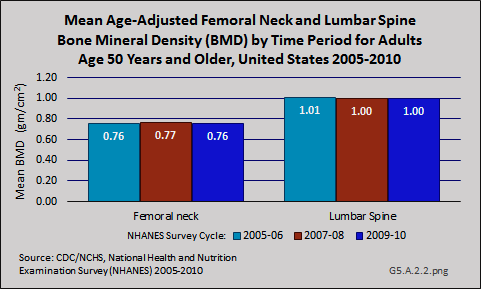
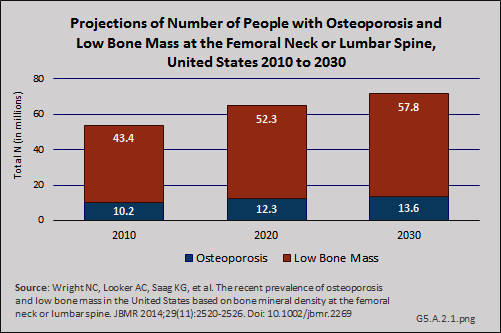
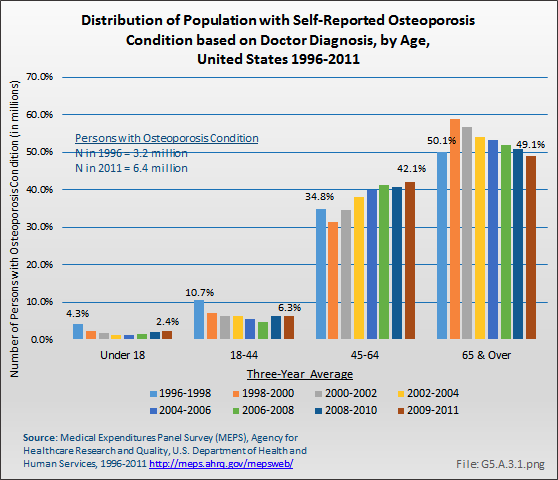
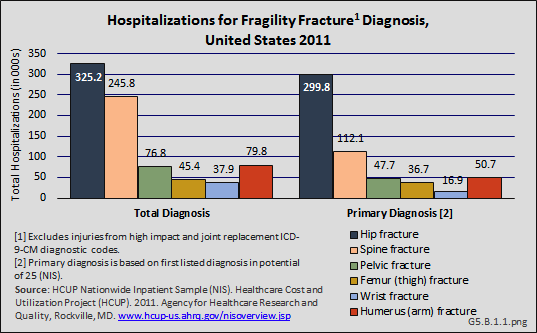
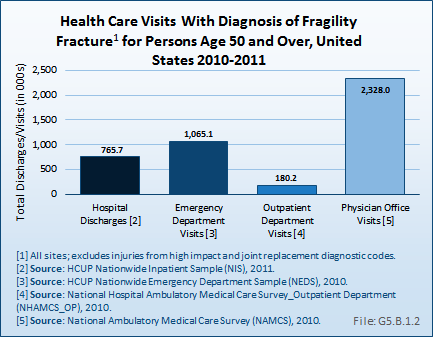
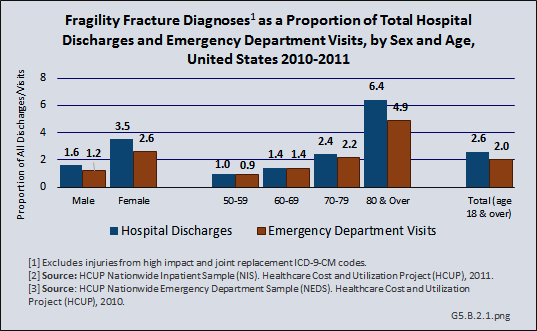
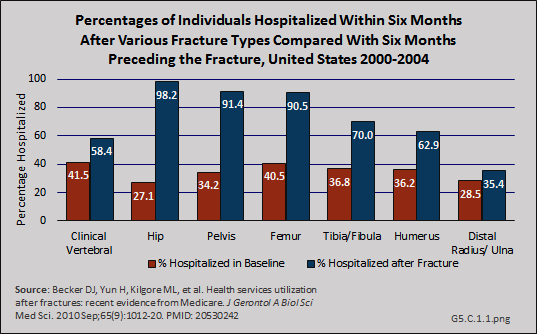
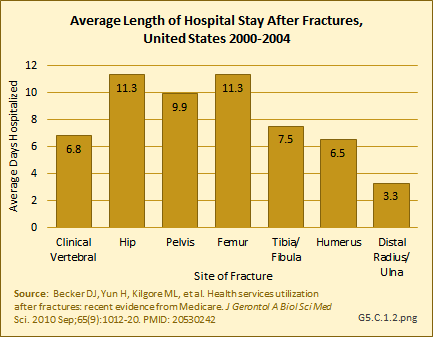
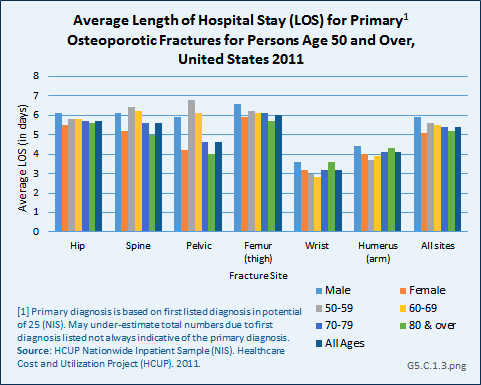
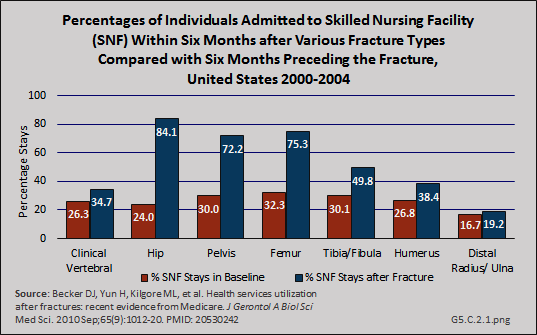
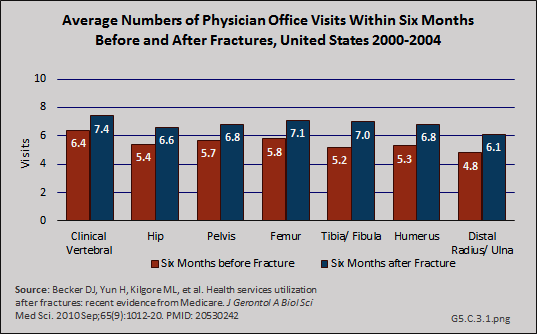
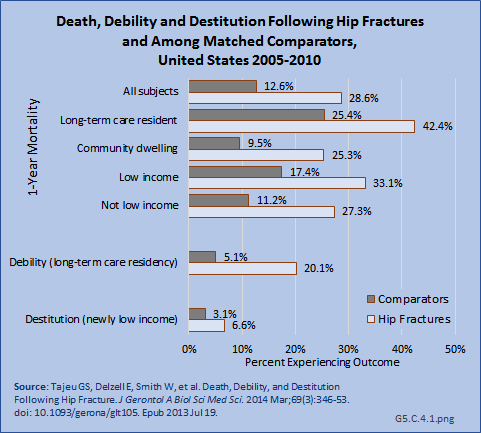
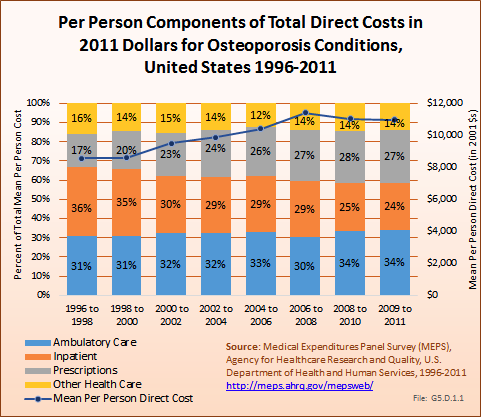
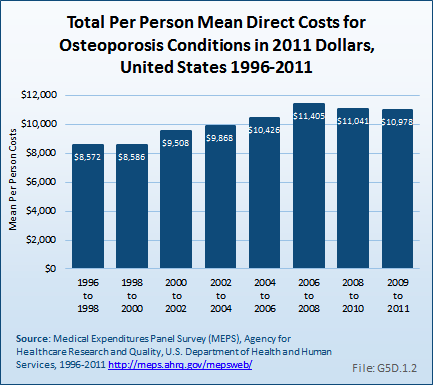
 Download as CSV
Download as CSV